Should I Join a Clinical Trial? A Lab and Eligibility Checklist
If you're thinking about joining a clinical trial, you may have heard that lab test reports, biomarkers, and genetic screening play important roles. What do these tests reveal, why do they matter, and what happens if your results don’t match the study’s criteria?

What is a Clinical Trial?
Clinical trials are essential to advancing medicine, they help researchers discover new treatments, understand disease behavior, and improve health outcomes for future patients. But before any new therapy can be approved and widely used, it must go through a series of highly structured clinical studies. These studies are designed not only to evaluate safety and effectiveness, but also to ensure that the right participants are matched with the right intervention.
Eligibility for a clinical trial isn't random. It's determined through a careful screening process that often includes blood work, biomarker analysis, imaging, and genetic testing. These diagnostic tools help researchers identify specific characteristics, such as a genetic mutation, protein expression, or organ function status, that determine whether a participant is a good fit for the study. Matching participants based on these insights improves safety, enhances results, and supports more personalized care.
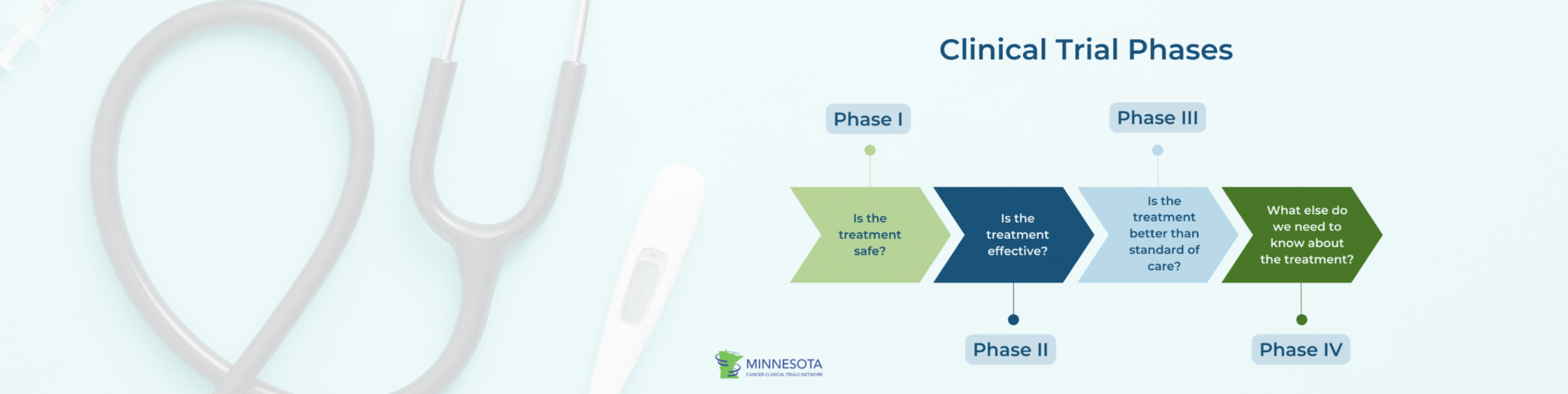
Clinical trials are typically divided into four phases, each with a specific purpose:
- Phase 1: Focuses on safety and dosage in a small group of participants.
- Phase 2: Examines effectiveness and side effects in a larger group.
- Phase 3: Confirms effectiveness, monitors side effects, and compares the intervention to standard treatments across large populations.
- Phase 4: Occurs after FDA approval to track long-term effects and real-world use (National Institutes of Health [NIH], 2024).
The phase of the study you're considering may affect what types of lab tests or genetic screening are required and how often they're performed. Each phase requires different types and frequencies of testing to ensure safety and appropriateness for participants.
The research staff describes the process, what to anticipate, and the way to prepare yourself in the trial. The research team is there to help you feel informed, prepared, and safe.
Real Stories, Real Impact: James and Joselyn's Paths to Clinical Trials
James, a 52-year-old father of two, was recently diagnosed with a rare form of lymphoma. Standard treatments offered limited success, so his oncologist mentioned a clinical trial. After a series of blood draws, a genetic test revealed that James had a mutation making him an ideal candidate for a targeted therapy. Today, he's not only responding well, but his progress is helping guide future treatment protocols. Stories like James's underscore how precision testing and eligibility screening can make a profound impact, not just in treatment selection, but in outcomes and future innovation.
Joselyn, a 36-year-old mother of one from Boston, had a different but equally powerful experience. Though healthy, her family history of breast cancer prompted her to undergo genetic testing, where she learned she carried a BRCA1 mutation. Rather than wait, Joselyn explored preventive strategies and enrolled in a clinical trial for a vaccine designed for high-risk individuals like her. The trial required multiple rounds of blood work and genetic screening, not only to confirm eligibility but to track how her immune system responded over time. Through her participation, Joselyn gained access to cutting-edge prevention tools and the peace of mind that came with frequent monitoring. Just as importantly, she became a vocal advocate in her community, raising awareness about genetic risk, clinical research, and the power of early action.
Together, James and Joselyn's stories highlight how lab results, genetic testing, and biomarker analysis can do more than determine eligibility, they can open doors to life-changing care, proactive planning, and new roles as empowered participants in medical discovery.
But before a trial can begin, each participant, whether seeking treatment or prevention, must go through a screening process to ensure the study is a safe and appropriate match. That's where the clinical trial eligibility check comes in. This detailed assessment uses lab tests, genetic analysis, and biomarkers to determine whether you qualify, and how a trial might fit into your unique health journey.
Eligibility screening may include:
→ Blood tests to determine the health of the kidneys, liver, and other organs
→ Genetic testing to determine inherited traits or mutations
→ Protein, hormone, or other molecular quantification by testing with biomarkers
→ Imaging tests such as CT (Computed Tomography) or MRI (Magnetic Resonance Imaging) scans
→ Physical examination and medical history review (Mayo Clinic, 2024)
These tests form the basis upon which your trial eligibility is determined and upon which the efficacy of the treatment under investigation is assessed. Now that you know what types of tests are involved in determining trial eligibility, you might wonder why so many detailed evaluations are necessary. Understanding the reasons behind these tests can help you feel more confident about the process and its role in keeping you safe and matched with the right study.
What Justifies the Order for These Tests?
Clinical trials require more than standard lab and diagnostic testing because these evaluations serve several vital purposes:
- First, they help ensure participant safety by identifying potential health risks before investigational treatments are administered, for example, blood tests can reveal impaired kidney function that might increase the risk of side effects (NIH, 2024).
- Second, they help researchers select the right participants, particularly when trials are designed for people with specific genetic mutations or disease subtypes, such as cancer treatments targeting tumors with certain biomarkers (American Cancer Society [ACS], 2024).
- Third, consistent participant profiles are essential to maintain the reliability of study results; including individuals with conditions that don't align with the study's criteria could skew the data.
- Finally, timing is critical, some tests must be performed right after diagnosis or within a narrow window, ensuring the treatment or intervention begins at the optimal moment for both patient benefit and study accuracy (FDA, 2024).
How to Prepare for Your Tests
Whether you're joining a clinical trial or undergoing routine lab work, preparation plays a critical role in ensuring accurate results, avoiding unnecessary delays, and protecting your safety. Most screening tests are straightforward and relatively painless, like blood draws, urine tests, imaging, or physical exams, but even small oversights can affect your eligibility or skew results. In clinical trials, lab tests are often used not just to monitor your current health, but to determine if you're a good fit for the study and whether it's safe to proceed with an investigational treatment. That means preparation isn't just about convenience, it's about getting the most accurate snapshot of your health.
Follow Fasting Instructions Carefully
Some blood tests, such as those measuring glucose, lipids (cholesterol, triglycerides), or insulin, require fasting for 8–12 hours. Even small snacks or flavored drinks can impact results. Water is typically allowed, but always confirm specific instructions with your care team ahead of time. If you're unsure whether a test requires fasting, ask, never assume.
Bring a Complete List of Medications and Supplements
Include all prescription medications, over-the-counter drugs, vitamins, supplements, and herbal products. Some substances may skew lab results or interact with investigational treatments. If you've recently changed or stopped a medication, let your care team know, even temporary changes can influence results.
Avoid Certain Foods and Beverages
Alcohol and caffeine can affect hydration, heart rate, and liver enzyme tests. Avoid them for at least 24 hours before your appointment. Also, foods rich in vitamin K (like leafy greens) may interfere with blood clotting or anticoagulant tests. Ask your care team whether any dietary restrictions apply to your particular tests.
Share Recent Health Changes
Let the clinical team know if you've had any recent infections, fever, inflammation, vaccinations, surgeries, or unusual symptoms, even if they seem minor. These can impact lab values or your eligibility. Don't downplay symptoms, clinical trials prioritize safety and need a full picture of your health.
Stay Well-Hydrated (Unless Instructed Otherwise)
Drinking plenty of water the day before and the morning of your blood draw helps keep veins plump and easy to access. Dehydration can make it difficult for the phlebotomist to draw blood and may affect certain lab results.
Bring Identification and Required Documents
Bring a government-issued ID and any documentation requested by the study team, such as:
→ Insurance information (if applicable)
→ Recent test results
→ Medical records
→ Vaccination history
Call ahead or check your trial portal to confirm what's needed (MedlinePlus, 2024).
Additional Tips:
- Dress in layers or wear short sleeves to allow easy access for blood draws or other procedures.
- Avoid intense exercise 24 hours before testing, as it may temporarily alter results like liver enzymes or creatine kinase (CK).
- Plan your schedule to allow rest after testing, especially if you're giving multiple samples or undergoing biopsies or imaging.

What Do the Results Mean?
Once your screening tests are complete, the clinical trial team will review the results with you to determine your eligibility and next steps. They'll assess whether you meet the study's inclusion criteria, which could involve factors like your age, diagnosis, genetic markers, and whether your lab values fall within the required range. They'll also look for any exclusion criteria, such as a coexisting diagnosis, abnormal test results, or recent treatments, that might interfere with the trial. Ensuring your results fall within normal or acceptable ranges is essential for your safety and the integrity of the study data. Occasionally, testing may uncover unrelated health issues; if that happens and you're not eligible for the trial, the primary investigator may refer you for follow-up care. If any results are inconclusive or borderline, you may be asked to repeat the tests, especially since certain results are only valid for a limited time (NIH, 2024).
Understanding Biomarkers and Genetic Testing
Biomarkers and genetic tests may sound technical, but they provide powerful insights that help tailor treatments to each individual. These tests provide critical insights into how your body is functioning, how your disease may behave, and which treatments or clinical trials are most likely to benefit you. Understanding these tools can empower you to make informed decisions and advocate for care that's truly personalized.
What Are Biomarkers?
A biomarker is a measurable indicator of what's happening in your body. It might be a substance found in your blood, tissue, or other fluids or even a genetic trait. Biomarkers provide critical data that researchers and doctors use to understand your health, predict how your disease may behave, and monitor how you respond to treatment.
Some examples are:
- PSA (Prostate-Specific Antigen): A test for prostate health. Elevated levels may indicate prostate inflammation or cancer.
- HER2 (Human Epidermal Growth Factor Receptor 2): An overexpressed protein in some breast cancers that can be targeted with specific therapies.
- HbA1c (Hemoglobin A1c): Reflects average blood sugar levels over time; crucial in managing diabetes and metabolic disorders.
- CRP (C-reactive protein): A marker of inflammation that can indicate infection, autoimmune activity, or cancer-related inflammation.
Why Are Biomarkers Important in Research and Clinical Trials?
In clinical studies, biomarkers are used for more than diagnosis—they guide personalized treatment strategies (FDA, 2024). Depending on your biomarker profile, you might:
→ Access targeted therapies designed to work with your specific biology
→ Avoid unnecessary treatments that are unlikely to be effective for your biomarker type
→ Be eligible for clinical trials focused on people with your biomarker or genetic mutation
Biomarkers help researchers:
→ Predict how well a specific treatment might work for you
→ Monitor your disease over time and catch signs of progression early
→ Fine-tune dosage so you receive the most effective and safest amount
What Is Genetic Testing?
While biomarkers give important clues about how your body is functioning right now, genetic testing takes a deeper look at your DNA, the genetic blueprint that influences your health, inherited traits, and risk for certain diseases.
Genetic testing examines your DNA to identify inherited mutations or changes that may affect how your body develops disease or responds to treatment. For example, detecting mutations in genes like BRCA1 or BRCA2 can help determine whether you're a candidate for specific targeted cancer therapies or preventative strategies.
Typically, genetic testing involves:
→ Providing a blood or saliva sample that contains your DNA
→ Sending your sample to authorized, certified laboratories for analysis
→ Protecting your genetic information under strict confidentiality and privacy laws
Understanding your genetic profile can offer insights that shape your treatment plan and open doors to personalized medicine options and clinical trials tailored to your unique genetics.
What If You Don't Qualify?
If you find out that you don't qualify for a specific clinical trial, it can feel disappointing, but it's important to remember that you still have options and potential advantages. You may be eligible for another trial, as there are many studies being conducted across the country. Your research team can help guide you toward other opportunities that may be a better fit. Additionally, the testing process itself can uncover early warning signs or potential health issues that you and your doctor can address together. These insights can open the door to conversations about how to proactively manage and maximize your overall health.
Final Thoughts:
Joining a clinical trial is a personal decision, one that can open the door to innovative treatments and contribute to discoveries that help others. Understanding the role of lab tests, biomarkers, and genetic screening gives you the power to ask informed questions and make confident choices. No matter where you are in your care journey, knowledge is your ally.
Talk to your care team today about clinical trials you may be eligible for. Use the checklist below to prepare for your next appointment and bring questions. Your preparation today could open the door to tomorrow’s treatments.
Clinical Trial Eligibility & Lab Testing Checklist
What to Know, What to Expect, and How to Prepare
☐ Clarify the Trial Framework
☐ Understand the Required Diagnostics
☐ Before You Go: How to Prepare for Screening Tests
☐ Bring to Your Appointment
☐ Key Questions to Ask the Research Team
☐ If You Don't Qualify
Sources:
American Cancer Society. (2024). Genetic testing for cancer. https://www.cancer.org/cancer/cancer-causes/genetics/genetic-testing.html
Food and Drug Administration. (2024). Biomarkers and surrogate endpoints. https://www.fda.gov/drugs/biomarker-qualification-program/biomarkers-and-surrogate-endpoints
Mayo Clinic. (2024). Clinical trials: What you need to know. https://www.mayoclinic.org/tests-procedures/clinical-trials/about/pac-20384678
MedlinePlus. (2024). Medical tests. https://medlineplus.gov/lab-tests/
National Institutes of Health. (2024). Understanding clinical trials. https://www.nih.gov/health-information/nih-clinical-research-trials-you
Diagnostic Equity Resources
Confused by your lab test results? Get your lab test questions answered about your lab test report.
This information is not a substitute for, nor does it replace professional medical advice, diagnosis, or treatment. If you have any concerns or questions about your health, you should always consult with a healthcare professional.
